Navigating the 2025 FDA Drug Approval Calendar: A Look Forward at Potential Breakthroughs and Challenges
Associated Articles: Navigating the 2025 FDA Drug Approval Calendar: A Look Forward at Potential Breakthroughs and Challenges
Introduction
On this auspicious event, we’re delighted to delve into the intriguing matter associated to Navigating the 2025 FDA Drug Approval Calendar: A Look Forward at Potential Breakthroughs and Challenges. Let’s weave attention-grabbing data and supply recent views to the readers.
Desk of Content material
Navigating the 2025 FDA Drug Approval Calendar: A Look Forward at Potential Breakthroughs and Challenges
![]()
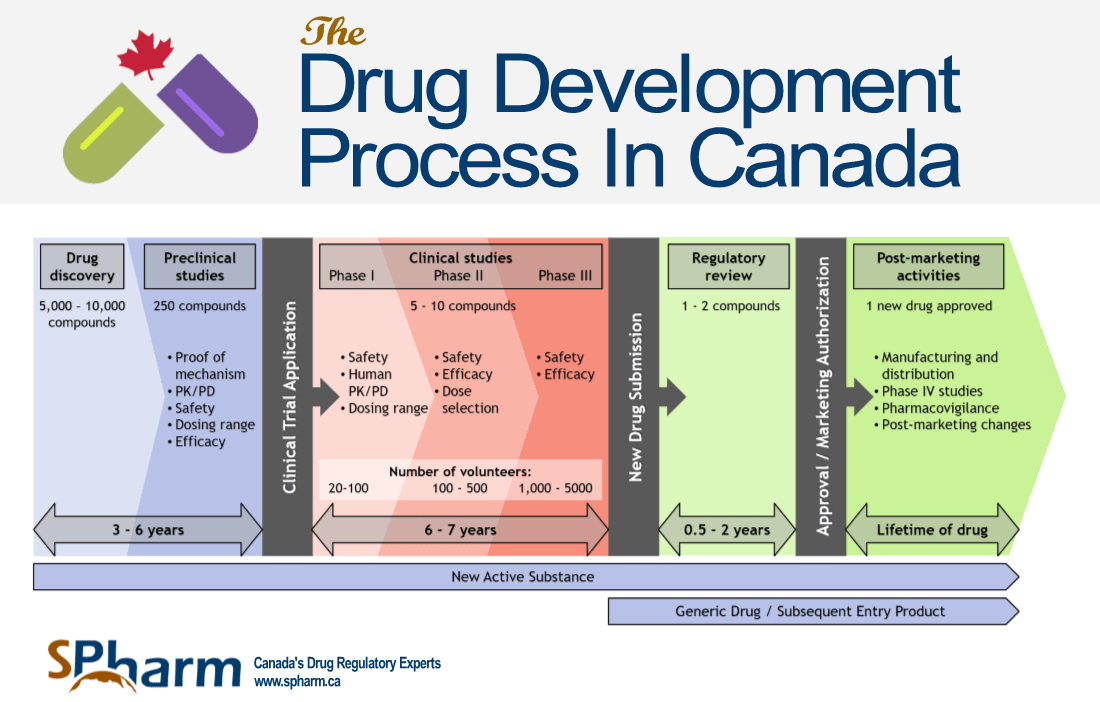
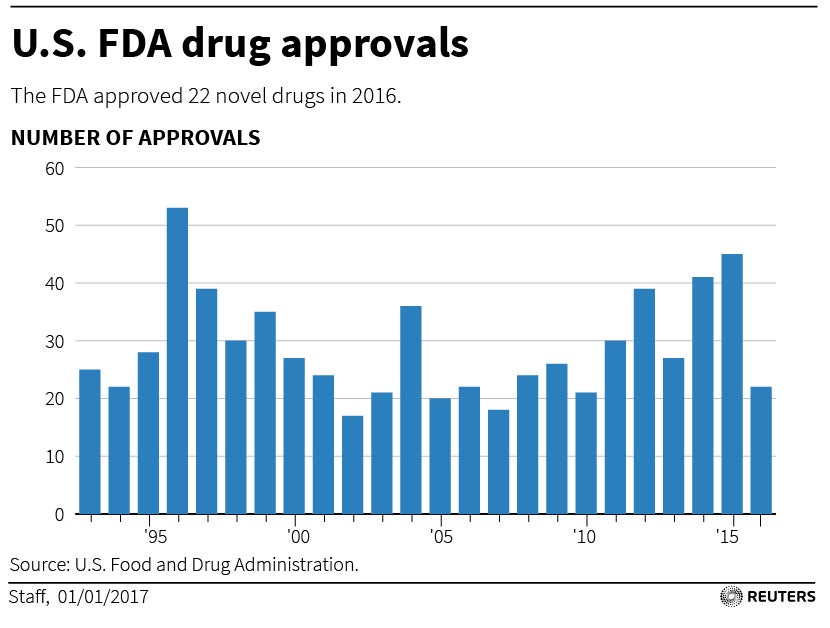
The Meals and Drug Administration (FDA) performs a pivotal position in safeguarding public well being by rigorously evaluating the protection and efficacy of latest medication earlier than they attain the market. Predicting the precise contents of the 2025 FDA drug approval calendar is unimaginable, as timelines are topic to alter based mostly on quite a few elements, together with medical trial outcomes, regulatory interactions, and unexpected circumstances. Nonetheless, by analyzing present medical trial pipelines, ongoing regulatory opinions, and historic FDA approval developments, we will speculate on potential areas of focus and anticipate a few of the key therapeutic areas which will dominate the 2025 panorama.
Predicting the Unpredictable: Challenges in Forecasting FDA Approvals
Forecasting FDA approvals is inherently difficult because of the inherent complexities concerned:
- Medical Trial Outcomes: The success or failure of a drug hinges completely on the outcomes of its medical trials. Sudden hostile occasions, inconclusive efficacy knowledge, or failure to fulfill pre-defined endpoints can considerably delay or derail the approval course of.
- Regulatory Hurdles: The FDA’s evaluation course of is rigorous, involving a number of phases of analysis and potential requests for added knowledge or modifications to the applying. Navigating these hurdles can result in unpredictable delays.
- Manufacturing and Provide Chain Points: Guaranteeing constant manufacturing high quality and establishing strong provide chains are vital for drug approval. Manufacturing challenges can create vital delays.
- Competitors and Market Dynamics: The aggressive panorama can affect FDA selections. If a number of firms are creating comparable medication, the FDA could prioritize the applying that demonstrates superior security and efficacy.
Potential Therapeutic Areas for 2025:
Regardless of these challenges, sure therapeutic areas are poised to see vital exercise in 2025 based mostly on present medical trials and ongoing regulatory opinions. These embrace:
-
Oncology: Most cancers stays a serious focus of pharmaceutical analysis and improvement. We are able to anticipate approvals for novel therapies concentrating on particular most cancers varieties, together with immunotherapies, focused therapies, and novel mixtures. This contains potential developments in:
- CAR T-cell remedy: Continued refinement and enlargement of this revolutionary most cancers remedy method to handle a wider vary of cancers.
- Immuno-oncology: New checkpoint inhibitors and mixture therapies concentrating on completely different immune pathways are prone to emerge.
- Focused therapies: Precision drugs continues to drive innovation, with new medication concentrating on particular genetic mutations or molecular pathways in numerous cancers.
-
Neurological Problems: The numerous unmet medical wants on this space are driving substantial funding in analysis. Potential approvals could embrace:
- Alzheimer’s illness: New disease-modifying therapies are extremely anticipated, although the problem of demonstrating medical efficacy stays vital.
- Parkinson’s illness: Advances in symptomatic remedy and disease-modifying approaches are prone to proceed.
- A number of sclerosis: New therapies concentrating on completely different illness mechanisms may emerge, bettering affected person outcomes.
-
Infectious Ailments: The continuing risk of rising infectious illnesses, coupled with the necessity for improved therapies for current infections, is driving analysis on this space. Potential approvals may embrace:
- Antivirals: New antiviral brokers concentrating on resistant strains of viruses, together with influenza and HIV, are extremely possible.
- Antibiotics: The pressing want for brand spanking new antibiotics to fight antimicrobial resistance is prone to result in approvals of novel brokers with completely different mechanisms of motion.
-
Uncommon Ailments: The growing deal with orphan medication and the event of focused therapies for uncommon illnesses are anticipated to end in a number of approvals. This contains potential breakthroughs in:
- Genetic issues: Gene therapies and different progressive approaches are prone to yield approvals for uncommon genetic illnesses.
- Metabolic issues: New therapies concentrating on particular metabolic pathways may emerge.
-
Cardiovascular Ailments: Whereas vital advances have been made in treating cardiovascular illnesses, ongoing analysis continues to deal with bettering outcomes and lowering mortality. Potential approvals could embrace:
- Novel anticoagulants: Improved anticoagulants with fewer uncomfortable side effects are prone to be developed.
- Coronary heart failure therapies: New therapies concentrating on the underlying mechanisms of coronary heart failure may emerge.
Elements Influencing the 2025 Calendar:
A number of elements past the precise therapeutic areas will form the 2025 FDA drug approval calendar:
- FDA Regulatory Priorities: The FDA’s strategic priorities, reminiscent of addressing unmet medical wants in particular areas, combating antimicrobial resistance, or advancing precision drugs, will affect which functions obtain precedence evaluation.
- Technological Developments: Advances in drug improvement applied sciences, reminiscent of synthetic intelligence and massive knowledge analytics, are accelerating the drug discovery and improvement course of, doubtlessly resulting in sooner approvals.
- Healthcare Coverage: Modifications in healthcare coverage, together with pricing and reimbursement rules, can have an effect on the business viability of latest medication and, consequently, the tempo of approvals.
- International Collaboration: Elevated worldwide collaboration in drug improvement can speed up the approval course of by sharing knowledge and sources.
Conclusion:
Whereas a exact prediction of the 2025 FDA drug approval calendar stays elusive, analyzing present medical trial pipelines and regulatory developments gives precious insights into potential breakthroughs. The areas of oncology, neurological issues, infectious illnesses, uncommon illnesses, and cardiovascular illnesses are prone to dominate the panorama, pushed by vital unmet medical wants and ongoing analysis efforts. Nonetheless, the unpredictable nature of medical trial outcomes, regulatory hurdles, and market dynamics implies that surprises are all the time attainable. The 2025 calendar will finally replicate the end result of years of analysis, improvement, and rigorous FDA evaluation, providing hope for sufferers awaiting new and improved therapies. Steady monitoring of medical trial progress, FDA bulletins, and business information will likely be essential for staying abreast of the evolving panorama of drug approvals in 2025 and past.


Closure
Thus, we hope this text has supplied precious insights into Navigating the 2025 FDA Drug Approval Calendar: A Look Forward at Potential Breakthroughs and Challenges. We thanks for taking the time to learn this text. See you in our subsequent article!
